No products in the cart.
CBDa Explained: Unique Uses and What Sets It Apart From CBD

Key Takeaways
- CBDa is an acidic precursor cannabinoid that turns into “activated” CBD through decarboxylation.
- CBDa is not psychoactive, nor will its use make you feel intoxicated.
- CBDa, like CBD, works with non-ECS receptors and may be more effective than CBD for certain health conditions.
Table of Contents
We cover CBD on our blog quite a lot (because we are a majority worker-owned farm that makes CBD products), but we don’t give much love to CBDa. That changes right now. For a long time, we believed that CBDa was merely the “inactive” form of CBD. But, with new research coming out, it may be that we gave the acidic cannabinoid an unfair shake.
In this post, we’ll explore CBDa, how it differs from CBD, and what new research is telling us about this much-overlooked cannabinoid.
What Is CBDa?
CBDa, short for cannabidiolic acid, is a naturally occurring compound found in hemp and cannabis. It is the acidic precursor of the much more well-known cannabinoid, CBD.
We find CBDa more in raw and unharvested hemp. That’s because after hemp is harvested, it starts to convert into CBD through a process called decarboxylation. More on that shortly.
Is CBDa Psychoactive?
CBDa is not psychoactive in the sense that you will not feel intoxicated after taking it. Like its successor, CBD, CBDa does not interact with your CB1 and CB2 receptors like THC does.
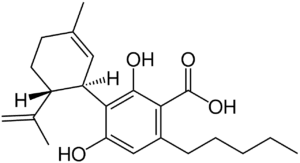
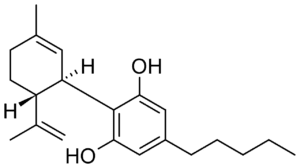
Chemical Structure
CBDa’s chemical structure is similar to that of CBD, save for one crucial addition. CBDa has a carboxyl ring group (COOH) attached to its benzoic acid ring. This one addition accounts for 13% of its molecular weight.
What’s The Point Of The Ring Group?
You might be thinking, “What’s the point of the ring group?”
The carboxyl ring group plays a crucial role in the chemical structure and stability of the CBDa molecule.
- Chemical Stability: The carboxyl group makes the cannabinoid in its acidic form more chemically stable in the plant. This stability is essential for preserving and protecting CBDa in the raw plant material.
- Molecular Structure: The carboxyl group contributes to CBDa’s overall molecular structure and polarity, affecting how it interacts with other molecules and its solubility in different environments.
Turning CBDa Into CBD
The carboxyl ring group is also important for another reason: we must get rid of it to turn our cannabidiolic acid into CBD. Luckily, the process is easy, though it is a mouthful.
To decarboxylate CBDa (or any other major cannabinoid precursor compounds), we only need to apply heat. When we expose CBDa to heat, whether through smoking, vaping, or in edible preparation, it rapidly drops its carboxyl ring and “activates.”
CBDa vs. CBD: More Different Than You May Think
Even though CBDa is the precursor to CBD, these two cannabinoids don’t share many similarities. In this section, we’ll cover three main areas where they diverge.
- ECS Interactions: CBD and CBDa are thought to interact differently with the body’s endocannabinoid system. CBD acts as a facilitator and ensures that our ECS operates more efficiently. When we consume CBDa, we know that it doesn’t have a strong affinity with our CB1 or CB2 receptors, but we don’t know how it interacts with the endocannabinoid system. As studies evolve and delve deeper, we expect to understand how this acidic form of cannabidiol interacts with our bodies.
- Consumption Methods: Because CBDa products can’t be exposed to heat before consumption, we have to consume them differently than CBD. While popular CBD methods include edibles, oils, topicals, and smokable flowers, you will usually see CBDa limited to tinctures, capsules, and raw cannabis juices.
- Potential Benefits: CBDa and CBD present many of the same potential benefits. One exciting difference between the two is CBDa’s affinity for the 5-HT1A serotonin receptors. In large doses, CBD does interact with these receptors, but CBDa seems to exhibit a higher affinity. It may mean that CBDa may be more effective than CBD when looking to self-medicate serotonin issues.
Benefits In The Works: Early CBDa Findings
If CBD research is still in its infancy, research into its acidic precursor is a newborn. Save for a few one-off studies, we know little about CBDa or its potential benefits. We’ll summarize what we do know below.
Observed Interactions
- Serotonin Receptors: Research indicates that CBDa might interact more significantly with serotonin receptors, particularly the 5-HT1A receptor. This interaction is thought to contribute to its potential anti-anxiety and anti-nausea effects.1
- COX-2 Inhibition: CBDa may also inhibit cyclooxygenase-2 (COX-2), an enzyme involved in inflammation, which could contribute to its anti-inflammatory properties independently of CB1 and CB2 receptor binding.2
Therapeutic Potential
- Anti-Inflammatory Properties: The carboxyl group in acidic cannabinoids may enhance their anti-inflammatory properties. Some studies suggest that CBDa can inhibit COX-2 enzymes more effectively than CBD, which can be beneficial in reducing inflammation.4
- Nausea and Anxiety Reduction: The presence of the carboxyl group in CBDa has been linked to potential anti-nausea and anti-anxiety effects. This may be due to the unique way it interacts with serotonin receptors (specifically 5-HT1A receptors) in the body.1
CBDa Explained: The Wrapup
CBDa isn’t just a precursor to be ignored any longer. It’s an essential cannabinoid in its own right, with unique benefits and uses that differentiate it from CBD. As research continues, we expect to learn more about it and other acidic cannabinoids.
If you’re interested in CBDa products, please let us know. The more inquiries we get about them, the more likely we are to develop them. In the meantime, please check out our lineup of full-spectrum CBD products fresh from our farm.
Sources
- Bolognini, D. et al. “Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation.” British journal of pharmacology vol. 168,6 (2013): 1456-70. doi:10.1111/bph.12043
- Takeda, Shuso et al. “Cannabidiolic acid as a selective cyclooxygenase-2 inhibitory component in cannabis.” Drug metabolism and disposition: the biological fate of chemicals vol. 36,9 (2008): 1917-21. doi:10.1124/dmd.108.020909
- Formato, Marialuisa et al. “(‒)-Cannabidiolic Acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research.” Molecules (Basel, Switzerland) vol. 25,11 2638. 5 Jun. 2020, doi:10.3390/molecules25112638
- Rock, E M, and L A Parker. “Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats.” British journal of pharmacology vol. 169,3 (2013): 685-92. doi:10.1111/bph.12162